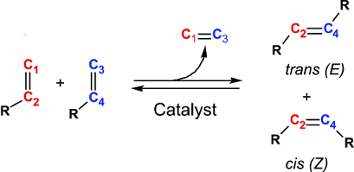
As illustrated in Figure 1, olefin metathesis reactions generally result in mixtures of two different geometric isomers, namely cis-olefins that carry the substituents (labeled by "R" in the figure) on the same side of the double bond, and trans-olefins featuring the substituents on opposite sides of the double bond. However, the target is often only one of the two isomers. The separation of the two isomers and work-up is impractical and costly, or sometimes even impossible, whenever cis-alkenes are the target products. This is the case for many biologically active natural products and pharmaceutical drugs.
| Figure 1. The olefin metathesis reaction provides a mixture of cis- and trans-isomers, also termed Z- and E-isomers. |

|
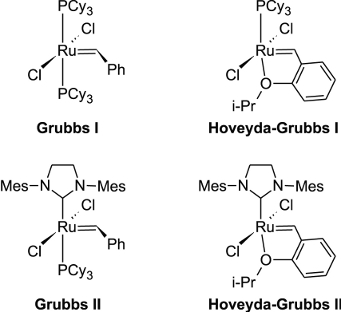
The most widely used olefin metathesis catalysts are the Grubbs ruthenium-based catalysts (see Figure 2), for which the arguably most serious limitation to the application of these catalysts is the lack of control of the ratio with which the two isomers (cis (Z) and trans (E)) of disubstituted olefin products are formed, with the thermodynamically favored product (the E-isomer) usually produced in considerable excess.
| Figure 2. Grubbs and Hoveyda-Grubbs-type ruthenium-based catalysts for olefin metathesis. |
|
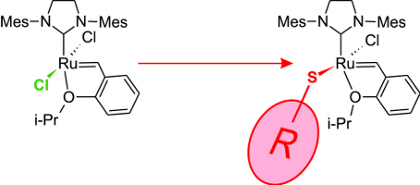
Intriguingly, by replacing one of the (small) chlorines by a sterically demanding thiolate ligand (see Figure 3), it has been possible to reach Z-selectivites above 90% (see Jensen et al. Int. Patent Appl. WO 2012032131, 2012, and Occhipinti et al. J. Am. Chem. Soc. 2013, 135, 3331-3334). Whereas this use of steric pressure to induce selectivity as well as the ligand substitution itself may seem straightforward, computational screening was necessary to identify a class of ligands able to offer high selectivity in combination with the otherwise standard Grubbs catalyst makeup.
| Figure 3. Cis-selective ruthenium-based catalysts containing a sterically demanding thiolate ligand, S-R. |
|